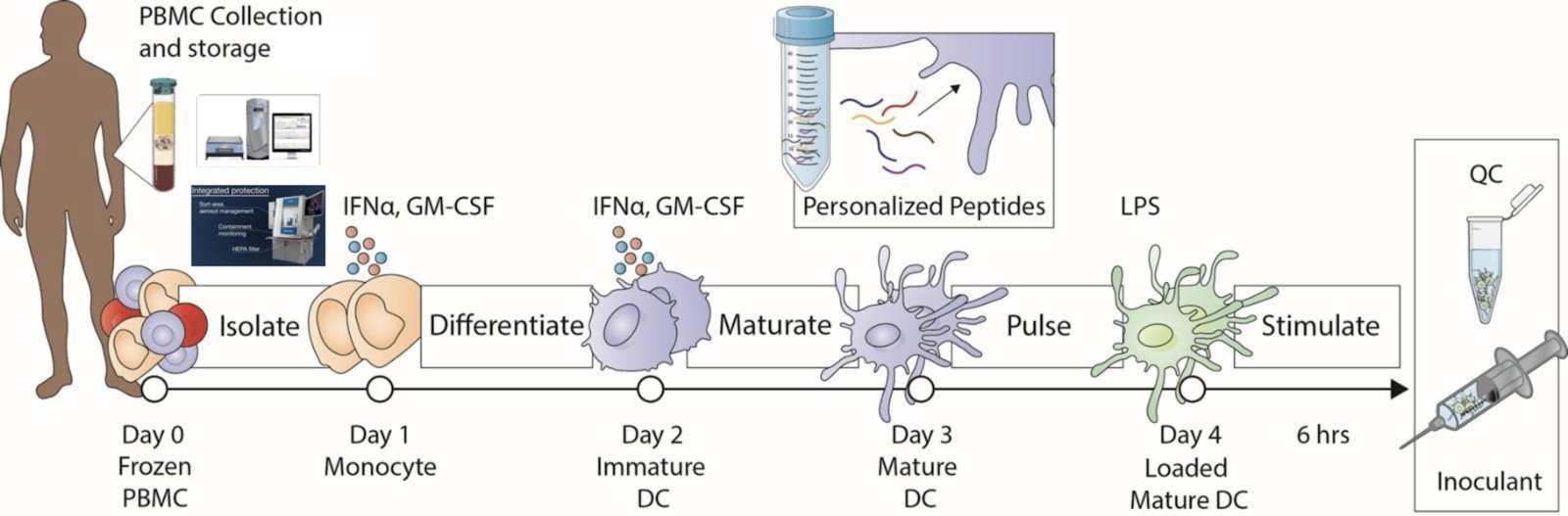
Personalized Medicine Process
The personalized portion of the treatment process involves several stages - extraction, testing, creation/selection of a personalized drug therapy, and treatment (injections).
PBMC = peripheral blood mononuclear cells; IFNa = interferon alpha; GM-CSF = granulocyte-macrophage colony stimulating factor; DC = dendritic cells; LPS = lipopolysaccharides; QC = quality control
Extraction
The process starts with a withdrawal of blood, from which DNA can be obtained for the testing and subsequent stages.
Testing
DNA is extracted and sequenced to determine the unique genotype that will guide the personalized treatment process..
Development of Personalized Therapy
The DNA testing will provide the data required to develop a personalized treatment plan and drug products. Proprietary software will determine and outline the most effective treatment plan for the individual.
Treatment
The personalized drug treatment will be administered to the patient in a number of injections, as per the treatment plan.
Platform
The hardware platform required for the process is built on a foundation of proven instrumentation technologies.
Sample Isolation
The sample isolation process uses commercially available instrumentation, meeting the quality requirements of an FDA approved system.
Sequencing
The DNA sequencing system is a platform with a long history of providing cost-effective DNA and RNA sequences for a variety of applications. A previous application for the sequencing hardware achieved FDA approval.